Improving the Lives of Patients with Cancer

Fast Facts

We are a commercial-stage, global pharmaceutical company with one FDA-approved drug in three oncology indications and three additional drug candidates in clinical development.

We have been working for over 10 years to advance research and development in cancer therapeutics.

Since the Company’s founding in 2008, we have grown into an international organization with corporate headquarters in Newton, Massachusetts with a presence in Israel and the European Union.

We are an immigrant female-founded biotech company where over 50% of our workforce and 25% of our leadership team are women, and over 35% of employees identify as part of a minority group.

We have been serving patients since 2012 when our novel drug selinexor first entered clinical trials and we continue to be inspired by these courageous patients and their families.

Our Mission
Everything we do at Karyopharm is driven by our mission to positively impact patient lives and defeat cancer. Our foundation is in our science, and we are the global leader in the inhibition of nuclear export.
We support a culture of innovation, courage, alignment and accountability, resiliency and energy (ICARE) with our employees and collaborators.


Our Blueprint For Success

Our Science
Science and innovation are the core principles by which we operate. We are pioneering novel science to harness each cell’s own natural defense mechanisms to prevent the initiation and growth of cancers. We are the leader in innovative science to advance cancer therapy by targeting the regulation of critical proteins that need to be located in a cell’s nucleus in order to function properly. This approach is synergistic with current anti-cancer treatments making it an ideal partner for future combination therapy approaches.
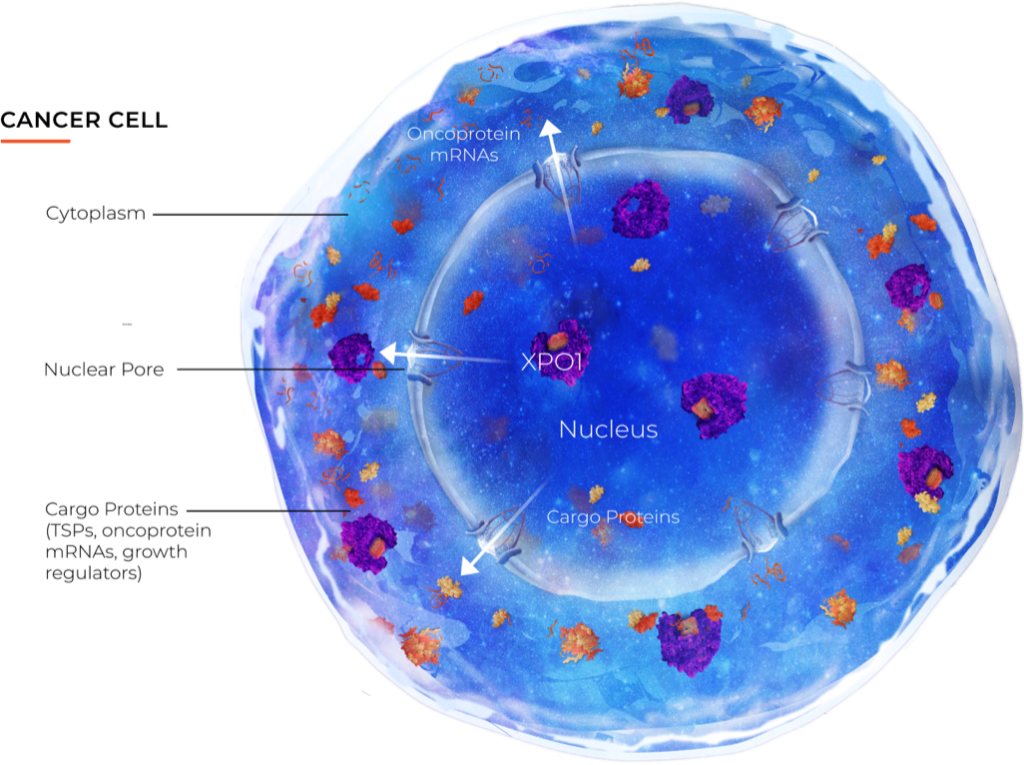

Our Approach
Our clinical development strategy for XPOVIO® has been to innovate with the purpose of helping difficult-to-treat patient populations and then expanding into larger patient groups and tumor types. The mechanisms of action of our novel nuclear export inhibitors target a foundational aspect of cancer biology which may allow them to be used against a very wide variety of different tumors. Our development began in hematologic malignancies and we have expanded our clinical trials into a variety of solid tumors.
We see our pipeline of products as a critical partner of choice to be combined with other cancer medicines in a host of different types of cancer.

Our Story
We began in 2008 with a vision of pioneering a new approach to treating patients with cancer and other serious diseases. Our novel approach to treating cancer involves targeting a cancer cell’s nucleus to prevent the development of a disease. For this reason, our name is based on the suffix ‘karyo’, which means nucleus.
In 2019, we received our first accelerated approval in penta-refractory multiple myeloma. We received a second accelerated approval in 2020 for relapsed or refractory diffuse large B-cell lymphoma (RR DLBCL).
We received an expanded approval for XPOVIO in December of 2020 to now include patients with multiple myeloma as early as first relapse.
We are undertaking numerous clinical trials with a bright future of potential FDA approvals yet to come.
Karyopharm works every day, innovating the science needed to improve the lives of patients with cancer.

Other Resources
To view our corporate press releases, please click here.
For media inquiries, please contact Sarah Connors, at sarah.connors@karyopharm.com